
Rapid onset of action in
as early as 10 minutes post dose13

Significant improvement in motor function post dose (P=0.009) (primary endpoint)13

Sustained effect post dose13
SPANSM-PD was a 12-week, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of INBRIJA for the treatment of OFF periods in patients with PD treated with CD/LD
SPAN-PD: Study Design11,13 (N=226)

WEEK
USE AT HOME11,13
-
Used at the start of an OFF period
-
Continued use of usual PD medications, including CD/LD
-
Self-administered as needed up to a maximum of 5 times during the waking day
IN-CLINIC DOSING AT WEEKS 0, 4, 8, AND 1211,13
-
Took morning CD/LD as usual and arrived in the ON state
-
Remained in clinic until they transitioned into an OFF period
-
Self-administered study drug at the start of the OFF period
UPDRS Part III* motor score at 30 minutes: change from predose OFF to 30 minutes post dose with INBRIJA 84 mg vs placebo
UPDRS Part III is a composite measure of 14 items designed to assess the severity of primary motor symptoms (eg, tremor, rigidity, bradykinesia, postural instability) in patients with PD. Disability level is evaluated using 27 assessments with a total score of 108 points.15
UPDRS, Unified Parkinson's Disease Rating Scale.
Select baseline criteria for the SPAN-PD study
-
CD/LD regimen not exceeding 1600 mg/day of levodopa
-
≥2 hours of OFF time per day
-
Hoehn and Yahr stage <2.5 (64.6% of patients)11
-
Mean UPDRS Part III motor scores in ON stage at screening: 14.9 for INBRIJA 84 mg and 16.1 for placebo
-
Patients with asthma, chronic obstructive pulmonary disease (COPD), or other chronic respiratory disease11
-
Use of apomorphine13
Mean Patient Baseline Charcteristics (N=226)11,13
| Age (range, 38-82 yr) | 63.0 yr |
|---|---|
| Gender (male)† | 75% |
| Ethnicity (white)† | 95% |
| Time since diagnosis | 8.0 yr |
| Number of OFF periods per day‡ | 3.4 |
| Duration of oral levodopa treatment | 6.5 yr |
| Daily oral levodopa dose | 830 mg |
| Number of daily oral levodopa doses | 5.1 |
†Not mean value.
‡Includes early morning OFF.
-
Dopamine agonists (57.5%)
-
Selective MAO-B inhibitors (38.9%)
-
Adamantane derivatives (19.9%)
-
COMT inhibitors (14.2%)
COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase B.
INBRIJA starts to work in as early as 10 minutes so patients can get back in charge of their day
UPDRS Part III score change from 0 to
60 minutes post dose at week 12

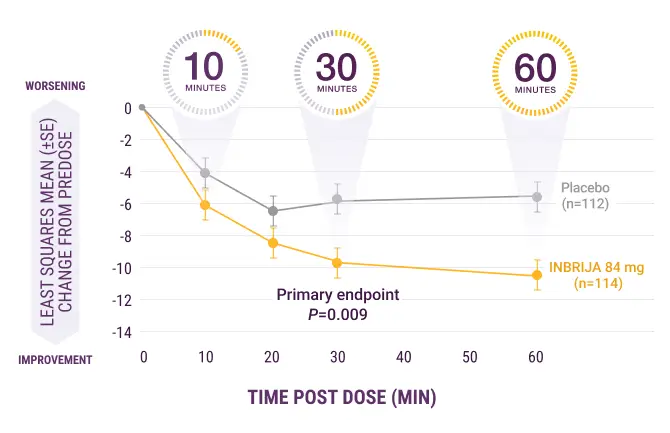 SE, standard error.
SE, standard error. Responder ON
A significantly greater proportion of patients taking INBRIJA 84 mg (58%) returned to ON state and sustained that ON through 60 minutes post dose vs placebo (36%; P=0.003) (first secondary endpoint)13
INBRIJA has an established safety profile
Adverse Reactions Occurring in ≥5% of INBRIJA-treated Patients and at a Higher Rate Than Placebo
| INBRIJA 84 mg (n=114) | PLACEBO (n=112) | |
| Cough | 15% | 2% |
| Upper respiratory tract infection | 6% | 3% |
| Nausea | 5% | 3% |
| Discolored sputum | 5% | 0% |
-
2% of 114 participants discontinued INBRIJA 84 mg due to cough
-
5% of participants taking INBRIJA 84 mg discontinued due to any adverse reactions, compared with 3% of patients taking placebo
-
Inhalation of INBRIJA can lead to coughing or the sensation of choking* at the time of administration
*Sensation of choking was identified during postapproval use of INBRIJA.


 Please see Full Prescibing
Please see Full Prescibing